Organosilicon refers to compounds that contain silicon-carbon bonds and have at least one organic group directly connected to a silicon atom. Compounds in which organic groups are connected to silicon atoms through oxygen, sulfur, nitrogen, etc. are often also considered organosilicon. compound.Inorganic silicon refers to compounds that do not contain silicon-carbon bonds, and all groups are connected to silicon atoms through oxygen, sulfur, nitrogen, etc.
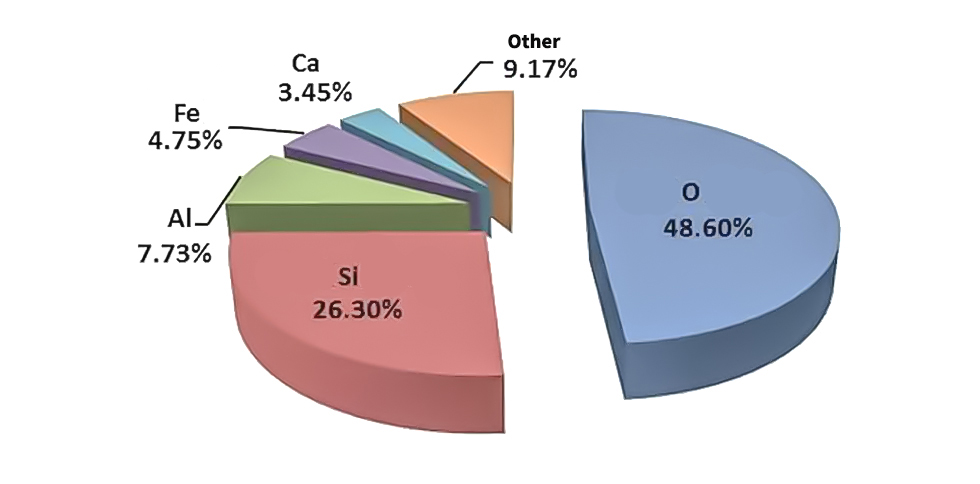
Figure 1: Distribution of silicon and other elements in the earth’s crust
Silicon is abundant in nature and is second only to oxygen in the earth's crust, accounting for approximately 26.3% of the mass of the earth's crust.In the thousands of years of human development, silicon atoms and their compounds have played an extremely important role.With the continuous development of science and technology, people's understanding of silicon-containing materials is also deepening, and the application scope of silicon-containing materials is constantly expanding.According to the historical context of human use of silicon-containing materials, human application of silicon-containing materials can be roughly divided into three stages: silicon-containing compounds (silica and silicates) are used as building materials; metallic silicon and its alloys are used Integrated circuits; silicone compounds are used in all aspects of human life.
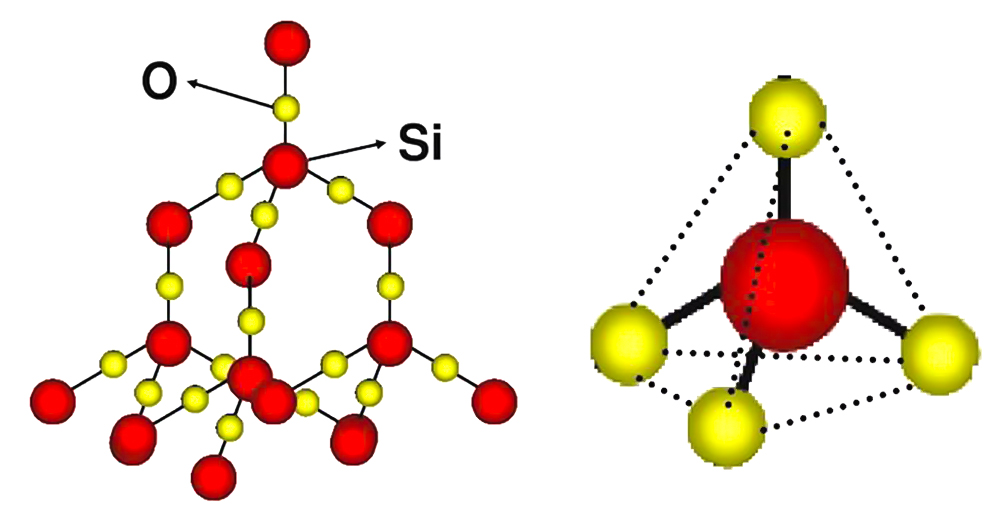
Spatial structure model of crystalline silica (picture from the Internet)
Since silicon is an oxygen-loving element, there is no free pure silicon in nature, but exists in the form of silicon-containing compounds.Silicon-containing compounds, mainly including silica and silicates, are the earliest silicon materials used by humans.At first, people applied silicon-containing materials, mainly using sand, soil, stone, etc. containing silica and silicates as building materials and production raw materials. Later, with the continuous development of science and technology, people prepared silicic acid, which increased the number of Types of silicon-containing compounds, and other uses of silicon-containing compounds such as silica, silicic acid, and silicates are also gradually being explored.
Left: crystal; middle: optical fiber; right: quartz glass (pictures from the Internet)
Naturally occurring silica is collectively called silica, and it mainly comes in two forms: crystalline and amorphous. Crystalline silica is also called quartz, which is in a colorless and transparent solid state. It is the main component of common decorations such as crystal and agate. Quartz, sand also contains tiny particles of quartz crystals.Amorphous silica mainly exists in diatomaceous earth, which is a porous, light and soft light yellow or light gray powder.Crystalline silica has a spatial network structure, which is very stable. Therefore, crystalline silica has a very high melting point and hardness, and is insoluble in water.Taken together, the main uses of silica are: contained in sand and soil and used as building materials; used as high-temperature-resistant quartz crucibles; used as quartz glass to make quartz watches and quartz clocks; and used as the main raw material for quartz and agate. Decorations and crafts; pure silica can be used to make optical fibers.

The color change process of color-changing silica gel after absorbing water (picture from the Internet)
Silicic acid is a white colloid that is weakly acidic and insoluble in water. It is easy to decompose when heated. It is also a silicon-containing compound.Compared with silica and silicate materials, people have applied silicic acid later, and it is mainly used as a carrier for silica gel desiccant and catalyst.

Left: glass; middle: cement; right: ceramics (pictures from the Internet)
Silicates are a general term for compounds composed of silicon, oxygen, and metal elements. They are the main components of rocks and soil. Except for sodium silicate and potassium silicate, other silicates are insoluble in water.Sodium silicate is a common silicate. It is a white solid and soluble in water. Its aqueous solution is called water glass. It is a colorless viscous liquid with viscosity and can be used to make adhesives.Items soaked in water glass are less likely to burn, so sodium silicate can also be used as a flame retardant.Silicates are also the most important components of the three traditional inorganic non-metallic materials: glass, cement and ceramics. So far, these three types of inorganic non-metallic materials still occupy an important position in people's production and life.JJBerzelius first prepared the chemical reaction equation of elemental silicon.
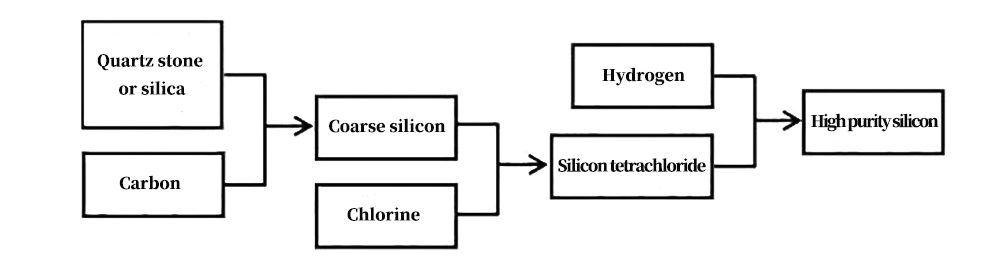
Schematic diagram of the preparation and purification process of crude silicon
Since early humans used sand, stone, soil and other materials containing silica and silicates as building materials, humans have been using silicon-containing compounds for thousands of years. However, it was not until the 19th century that people prepared elemental silicon. , and began to explore its potential applications.In 1824, the Swedish chemist JJ Berzelius reduced potassium fluorosilicate with potassium metal and obtained elemental silicon for the first time. Since then, the development of silicon materials has entered a new stage, with elemental silicon as the Various types of basic materials and products are constantly emerging.

Left: Integrated circuit; Right: Solar cell (pictures from the Internet)
Silicon (Si) comes in two forms: crystalline silicon and amorphous silicon. Crystalline silicon is gray-black and amorphous silicon is black.Silicon element has a very high melting point, boiling point and very high hardness. It is insoluble in water, nitric acid and hydrochloric acid, soluble in hydrofluoric acid and alkali, and has a metallic luster.Silicon is the 14th element in the periodic table. It is at the transition position between metals and non-metals. Therefore, its electrical conductivity is between conductors and insulators, and it is a very good semiconductor material.High-purity silicon can be used for chips, semiconductor materials (integrated circuits, transistors, etc.), solar cells, special-purpose alloys, and silicone production.
Friedel and Craft’s chemical reaction equation for the synthesis of the first organosilicon compound containing Si-C bonds (tetraethylsilane)
Whether it is silicon-containing compounds such as silica, silicic acid, silicate, or high-purity silicon, they are all used in people's production and life in the form of inorganic substances. The application scope of these inorganic silicon materials is relatively small, and After long-term development, its application potential has gradually been exhausted.The emergence of organosilicon compounds has created a new development path for the development and utilization of silicon-containing materials, adding a wide variety of silicon-containing materials and products, and rapidly expanding the application scope of silicon-containing materials.
Compounds that contain silicon-carbon bonds (Si-C) and have at least one organic group directly connected to a silicon atom are collectively called organosilicon compounds.It is customary to regard compounds in which organic groups are connected to silicon atoms through oxygen, sulfur, nitrogen, etc. as organosilicon compounds.Among them, polysiloxanes composed of silicon-oxygen bonds (-Si-O-Si-) as the skeleton have the most varieties and are the most widely used.Humans have only used organosilicon compounds for a short time. In 1863, French scientists Friedel and Craft heated silicon tetrachloride and diethyl zinc in a sealed tube to 160°C. This gave birth to the first organosilicon compound containing Si-C bonds - tetraethylsilane.Since then, a variety of organosilicon compounds have been manufactured and widely used in people's daily lives.
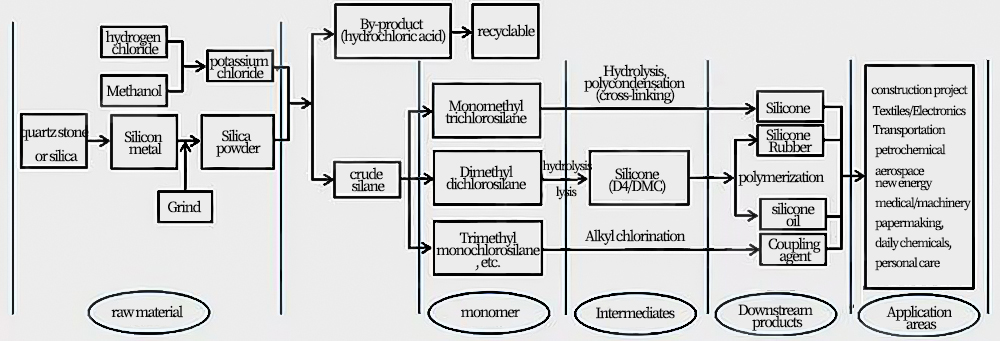
Schematic diagram of silicone industry chain
The organic silicone industry chain is very large, mainly including production raw materials, silicone monomers, intermediates, silicone oil, silicone resin, silicone rubber, silane coupling agents and other silicone downstream products, as well as new products and new products made from the deep processing of these downstream products. Materials and other parts, the production process is very complex.
Silicone itself is an emerging high-performance material with excellent temperature resistance, weather resistance, electrical insulation, physiological inertness, low surface tension and low surface energy. Therefore, it has great scientific research value and application potential. It provides material foundation and technical support for the entire materials industry, and is known as "industrial monosodium glutamate" and "technological development catalyst".It can be expected that for a long period of time in the future, silicone materials will continue to be used in construction engineering, textiles, electronics, transportation, petrochemicals, aerospace, new energy, medical care, machinery, papermaking, daily chemicals and personal care products. Areas such as people’s clothing, food, housing, and transportation serve human life.
Silicon, which belongs to the same main group as carbon on the periodic table of elements, has an important impact on human production and life. Silicon-containing inorganic compounds and silicon elements are the protagonists of inorganic non-metallic materials. Silicone polymers are used in people's daily life. All aspects of life.Therefore, understanding the "evolution" of silicon atoms from inorganic compounds and elements to organic silicon will help understand the historical context of human use of silicon-containing materials, and help scientific and technological workers gain more enlightenment from this process. Develop more silicon-containing materials and products that serve people's production and life.