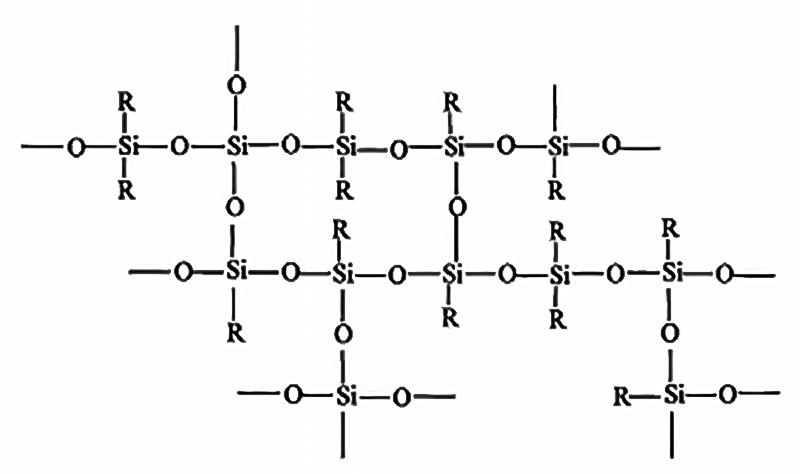
Organosilicon, that is, organosilicon compounds, refers to compounds that contain Si-C bonds and at least one organic group is directly connected to the silicon atom. It is customary to refer to those compounds that connect the organic group to the silicon atom through oxygen, sulfur, nitrogen, etc. The linked compounds are also referred to as organosilicon compounds. Among them, polysiloxane, which is composed of silicon-oxygen bonds (-Si-O-Si-) as the skeleton, is the most numerous, most deeply researched, and most widely used type of organosilicon compounds, accounting for more than 90% of the total usage.
Silicone materials have a unique structure:
(1) Sufficient methyl groups on Si atoms shield the high-energy polysiloxane main chain;
(2) C-H is non-polar, making the intermolecular interaction very weak;
(3) The Si-O bond length is longer and the Si-O-Si bond angle is large.
(4) Si-O bonds are covalent bonds with 50% ionic bond characteristics (covalent bonds have directionality, ionic bonds have no directionality).
2. Mainstream silicone products include the following four categories:
Silicone resin is a thermosetting plastic, and one of its most outstanding properties is excellent thermal oxidation stability. After heating at 250°C for 24 hours, the weight loss of silicone resin is only 2% to 8%. Another outstanding property of silicone is its excellent electrical insulation properties, which can maintain its good insulation properties over a wide temperature and frequency range.
Silicone resins can be roughly divided into several categories according to their main uses and cross-linking methods: silicone insulating paint, silicone coatings, silicone plastics and silicone adhesives.
2).Silicone rubber
Silicone rubber refers to a rubber whose main chain is alternately composed of silicon atoms and oxygen atoms. Silicon atoms are usually connected to two organic groups. Ordinary silicone rubber is mainly composed of methyl-containing silicon chains and a small amount of vinyl. The introduction of phenyl groups can improve the high and low temperature resistance of silicone rubber, and the introduction of trifluoropropyl and cyano groups can improve the temperature resistance and oil resistance of silicone rubber. Silicone rubber is one of the important products of silicone polymers and is widely used because of its excellent high and low temperature resistance. Silicone rubber is mainly divided into high temperature mixed silicone rubber, room temperature vulcanized silicone rubber, silicone gel, and foam silicone rubber.
3).Silicon monomer
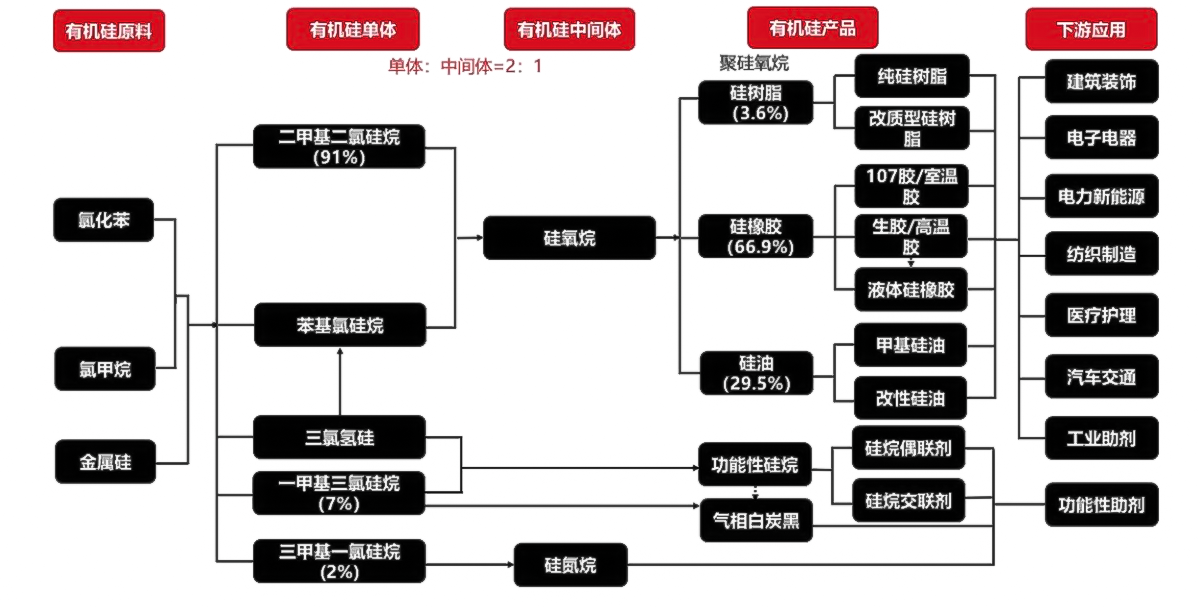
There are many silicone products, including tens of thousands of brands and more than 5,000 commonly used ones. However, its raw materials include several monomers, especially dimethyldichloride, which accounts for more than 90% of all monomers. Next is phenyl chloride.
1). Silicone resin
Silicone resin is a highly cross-linked network-structured polyorganosiloxane, usually made of methyltrichlorosilane, dimethyldichlorosilane, phenyltrichlorosilane, diphenyldichlorosilane or methylphenyl Various mixtures of dichlorosilane are hydrolyzed in the presence of organic solvents such as toluene at lower temperatures to obtain acidic hydrolysates. The initial products of hydrolysis are mixtures of cyclic, linear and cross-linked polymers, often containing a considerable number of hydroxyl groups. The hydrolyzate is washed with water to remove the acid, and the neutral primary condensation polymer is thermally oxidized in the air or further polycondensed in the presence of a catalyst, and finally forms a highly cross-linked three-dimensional network structure.
In view of the above characteristics, silicone resin is mainly used as insulating paint (including varnish, enamel, color paint, impregnating paint, etc.) to impregnate H-class motors and transformer coils, and is used to impregnate glass cloth, glass cloth silk and asbestos cloth to make motor covers. Tubes, electrical insulation windings, etc. Using silicone insulating paint to bond mica can produce large-area mica sheet insulation materials, which can be used as the main insulation of high-voltage motors. In addition, silicone resin can also be used as heat-resistant and weather-resistant anti-corrosion coatings, metal protective coatings, waterproof and moisture-proof coatings for construction projects, release agents, adhesives and secondary processing into silicone plastics for use in the electronics, electrical and defense industries. On the market, it is used as semiconductor packaging materials and insulating materials for electronic and electrical components.
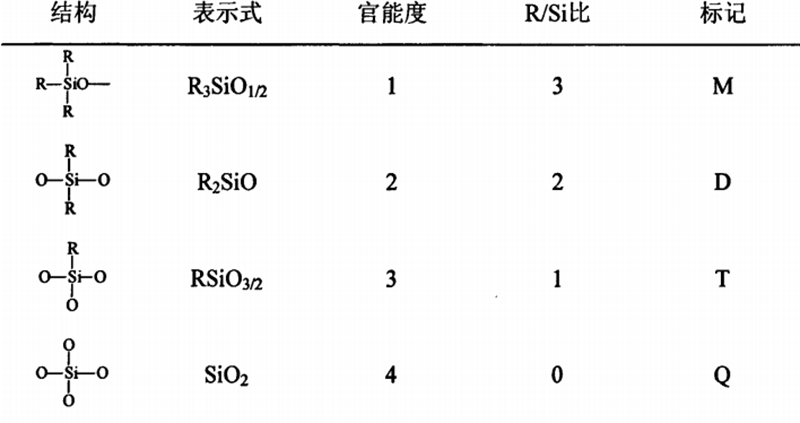

Silane, trimethylfluorosilane, ethyl and propyl chlorosilane, vinyl chlorosilane, etc. The monomer production process of silicone resin is complex and the process is long.
4).Silicon oil
Silicone oil is a kind of chain polysiloxane with different degrees of polymerization. Usually the organic groups are methyl groups, which are called methyl silicone oils. Common organic groups include C group, phenyl group, hydrogen group, etc. Generally, silicone oil is colorless or slightly yellow, odorless, non-toxic and non-volatile. Non-corrosive. The viscosity increases as the molecular weight increases, so there are silicone oil products of different viscosities.